KNO3 (Potassium Nitrate)
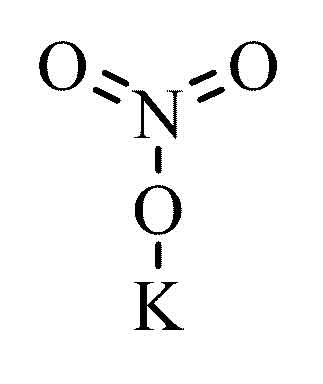

The Chemistry
Potassium nitrate is one of the main ingredients in gunpowder. Typical black powder is composed of 75% potassium nitrate, 15% charcoal, and 10% sulfur.
Potassium nitrate oxidizes charcoal and sulfur, which burn fiercely in an exothermic reaction which releases carbon dioxide and nitrogen gas. Here is a simplified reaction of gunpowder.
The History
Potassium nitrate is also known as nitre, or saltpeter. It comprises more than half of the chemicals in gunpowder, with the other part including charcoal and sulfur. Perhaps the most interesting example of nitre in history is in the American Civil War.
At the beginning of North-South hostilities in April 1861, both the Union and the Confederacy realized that they would need more nitre in order to win a prolonged war. When the Union first blockaded the Confederacy from importing Indian nitre from Britain, the desperate south responded by starting the Nitre Bureau in an all-out campaign to find domestic sources of nitre. At first, the South could only produce 500 pounds of nitre a day from caves in Kentucky and Tennessee, but eventually they were able to produce a ton a day with extensive mining in Texas.
The North had more productive and cost effective methods of obtaining nitre than the South. With the help of chemical magnate Henry DuPont and his son Lammot DuPont, the North imported 2 million pounds of nitre for the U.S. Navy.
The efficient production of nitre was key in the success of the Union in winning the Civil War.
