R-C9H11N2O4S (Penicillin)
The Chemistry
A lot of how Penicillin works is more related to biology than chemistry, so here is a simple breakdown:
An essential component of bacterial cell walls is a complex molecule called peptidoglycan. Penicillin prevents bacteria from successfully producing it. Here's a flow chart I made.
The History

Alexander Fleming.
Penicillin, the first antibiotic, was discovered by Alexander Fleming in 1928. After being studied in the UK and US, doctors in military hospitals began to use it to treat soldiers in WWII. Check out the stats.
Death from disease or wounds
16.5 per 1,000 (WWI) -> 0.6 per 1000 (WWII)
Death rate for soldiers admitted to hospitals
8% (WWI) -> 4% (WWII)
Penicillin Units made by US companies per month
650 BILLION!
Despite the success of penicillin in World War II, it is in no way a panacea for all diseases. Since its development many common pathogens such as tuberculosis have developed resistance, and its complex protein nature can trigger allergic reactions in some people.
KNO3 (Potassium Nitrate)
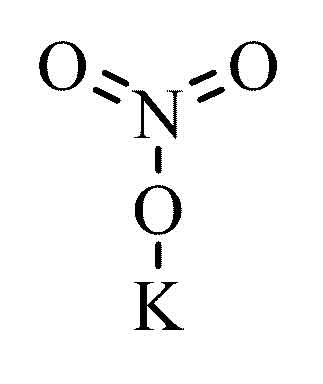

The Chemistry
Potassium nitrate is one of the main ingredients in gunpowder. Typical black powder is composed of 75% potassium nitrate, 15% charcoal, and 10% sulfur.
Potassium nitrate oxidizes charcoal and sulfur, which burn fiercely in an exothermic reaction which releases carbon dioxide and nitrogen gas. Here is a simplified reaction of gunpowder.
The History
Potassium nitrate is also known as nitre, or saltpeter. It comprises more than half of the chemicals in gunpowder, with the other part including charcoal and sulfur. Perhaps the most interesting example of nitre in history is in the American Civil War.
At the beginning of North-South hostilities in April 1861, both the Union and the Confederacy realized that they would need more nitre in order to win a prolonged war. When the Union first blockaded the Confederacy from importing Indian nitre from Britain, the desperate south responded by starting the Nitre Bureau in an all-out campaign to find domestic sources of nitre. At first, the South could only produce 500 pounds of nitre a day from caves in Kentucky and Tennessee, but eventually they were able to produce a ton a day with extensive mining in Texas.
The North had more productive and cost effective methods of obtaining nitre than the South. With the help of chemical magnate Henry DuPont and his son Lammot DuPont, the North imported 2 million pounds of nitre for the U.S. Navy.
The efficient production of nitre was key in the success of the Union in winning the Civil War.
C14H9Cl5 (DDT)

The Chemistry
DDT is a cheap and effective insecticide. In insects, DDT opens sodium ion channels, which then fire spontaneously and causes spasms and death.
One of the concerning properties of DDT is that it does not break down in the environment or an organisms. It has low solubility in water and high solubility in fats. Thus, when animals ingest DDT, it mixes with the fat and eventually builds up in their fat tissue.
The History
DDT was first created in Germany in 1874 and later developed by Paul Müller in the 1930s. In World War II, Allied soldiers used DDT to control malaria, typhus, body lice, and bubonic plague. After the war, agricultural industries applied DDT as a pesticide, and DDT was a large part of the World Health's Organization's 1955 push to end malaria.
DDT was used as an insecticide in large scale agriculture.
In 1962, Rachel Carson published her famous Silent Spring, which illuminated the dangers of pesticides such as DDT. Laboratory experiments have shown that DDT causes cancer, mutations, and birth defects, and DDT was banned in the US in 1972 by the Environmental Protection Agency. However, DDT is still used in South America, Africa, and Asia.
For more info, the National Pesticide Information Center has a great fact sheet about DDT:
http://npic.orst.edu/factsheets/ddtgen.pdf
NH3 (Ammonia)

The Chemistry
Ammonia has two major applications: fertilizer and explosives.


Of the nutrients necessary for plant growth, nitrogen is quite hard to obtain. Though air is 78% nitrogen, it must be fixed into a bio-available form through either natural or man-made processes. The development of the Haber process was so significant that it is estimated that fertilizer from ammonia produced by the Haber process helps sustain one-third of the Earth's population, and half the protein within human beings is made of nitrogen originally fixed using the Haber process.
As for explosives, the Haber-Bosch process allows ammonia to be oxidized and converted into nitric acid, which is the basis of explosives such as ammonium nitrate, nitro-glycerine, and trinitrotoluene (TNT). According to the International Nitrogen Initiative, it is responsible for the death of 150 million people.
The International Nitrogen Initiative also has more information about how ammonia is "the substance that changed the world."
Check it out: http://www.ini-europe.org/node/16
The History
World War I was a major application for chemical explosives derived from ammonia formed by the Haber-Bosch process. In 1910-1911, Bosch developed double-tubed hydrogen-resistant converters, which eventually led to the industrial conversion of ammonia to nitric acid, a main component of explosives used in the German military. Ammonia was first manufactured industrially in the BASF's Oppau plant in Germany, producing up to 20 tons of ammonia per day.
About Haber and Bosch: (I found this interesting because neither lived particularly happy lives)

Fritz Haber Carl Bosch
Haber is known as the "father of chemical warfare." Haber won the Nobel Prize in Chemistry in 1918. He did not mention the role of ammonia in chemical warfare in his acceptance speech at the Swiss Nobel Academy. Haber's wife, who opposed his work in chemical warfare, committed suicide in 1915. Haber left Germany following Hitler's rise to power and died of heart failure in 1934.
Bosch won the Nobel Prize in Chemistry in 1931. Bosch was a victim of depression and died in 1940.
C9H8O4 (Aspirin)
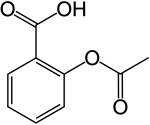

The Chemistry
The History

Felix Hoffman. Aspirin produced by Bayer.
Aspirin is now the most common drug in household medicine cabinets.
Check out the aspirin website, it literally has everything.
http://aspirin.com/scripts/pages/en/home.php
Sources
Last, J. M. (2002). Penicillin. In L. Breslow (Ed.), Encyclopedia of Public Health (Vol. 3, p. 898). New York: Macmillan Reference USA.
Kinard, Jeff. "Nitre and Mininig Bureau: American Civil War." World at War: Understanding Conflict and Society.
Neumann, Caryn E., and John Waksmundski. "Fritz Haber." World at War: Understanding Conflict and Society.
"DDT." United States Geography.
Griswold, M. "DDT." Life Sciences. Marshall Cavendish Digital, 2014. Web. 03 November 2014.
Vaclav Smil: Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production
Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production by Vaclav Smil
Isis, Vol. 93, No. 2 (June 2002), pp. 329-330


